Our platform

Our technology platform enables the production of high-demand molecules that emit radically fewer — or no — greenhouse gases at a cost that enables adoption in the short-term. Our commercial solutions are the culmination of more than 30 years of plasmonics and nanotechnology research out of Rice University.
Our technology is an extensible platform that can be tuned to produce molecules without significant reinvestment in additional equipment or retooling.
It consists of two pieces — our Rigel™ photoreactor and proprietary photocatalyst — that enable light-driven chemical reactions at unprecedented efficiency.
A new era for chemical reactors
Rigel photoreactors are designed from basic, affordable materials. They feature a photocatalyst-filled sleeve surrounded by a light box. Each reactor is fully contained in an outer shell. Banks of these reactors can be stacked to offer flexible installation sizes ranging from 1 ton of product per day to 100 tons or more per day.
When the Rigel is switched on, photons from light activate the photocatalyst in the heart of the reactor, triggering trillions of reactions per second to produce affordable, low-carbon-intensity chemicals. By simply switching out the catalyst and feeds, the same reactor can produce hydrogen and many other high-value molecules. It can also be used to convert harmful greenhouse gases into sustainable fuels and renewable methanol.
When using renewable electricity to power the light sources, Syzygy’s Rigel reactors are capable of producing zero-emissions hydrogen, low-carbon hydrogen, and other chemicals, while dramatically reducing the cost of production compared to other low-carbon technologies.
Learn how we use light to drive chemical reactions
The photocatalysts that unlocked a sustainable future.
The problem with today’s catalytic reactions is that they are driven by fossil-fuel-derived heating processes which emit significant volumes of greenhouse gases. Only by reducing reliance on conventional industrial heating can we ever hope to reduce the carbon intensity of the chemical industry. Syzygy’s proprietary photocatalyst provides a clear pathway for replacing combustion and eliminating emissions associated with powering chemical manufacturing.
Developing a photocatalytic process to shift our reliance away from thermal energy has been a goal for many research institutions and governments for more than 50 years. However, early semiconductor-type photocatalysts such as titanium dioxide had limited light absorption and surface chemistry profiles with subsequently low efficiencies and real-world applications. And conventional catalysts aren’t an option because they are not photo-reactive.
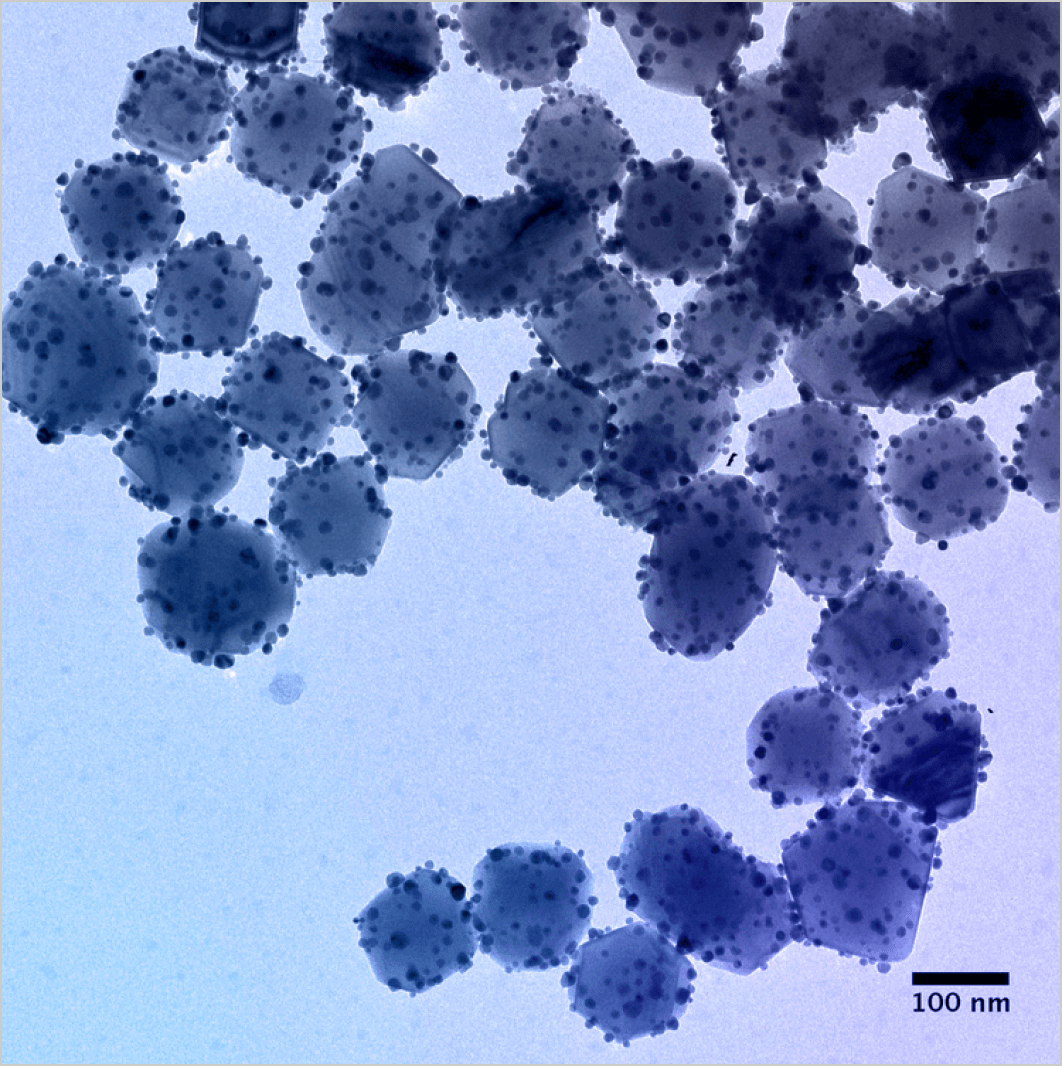
The breakthrough came when Rice University researchers embedded a nanoparticle of a traditional catalyst material into the surface of a larger light-harvesting plasmonic nanoparticle.
This two-part nanoparticle structure is referred to as an antenna-reactor. This antenna-reactor complex dramatically increased photocatalytic efficiency, making commercial applications for photocatalytic processes a reality for the first time. The antenna-reactor provides more efficient capture and transfer of light energy to the reactive sites on the catalyst, effectively replacing the need for thermal energy from the combustion of fossil fuels with light. Through further experimentation, researchers realized they could replace traditional rare, expensive catalytic metals like ruthenium with abundant, affordable light-reactive metals like iron. This further reduces production costs and the chemical industry’s consumption of rare metals.
Our current solutions include Ammonia e-Cracking™ for Hydrogen, Steam Methane e-Reforming for Hydrogen, and GHG e-Reforming™ for Syngas and Fuels.
We maintain a robust development pipeline and will continue to target reactions across the chemical industry value chain. And our technology and processes are compatible with existing infrastructure and workflows, further accelerating timelines for reaching lower-carbon-intensity goals.