Light-driven chemistry. More sustainable and more cost-efficient.

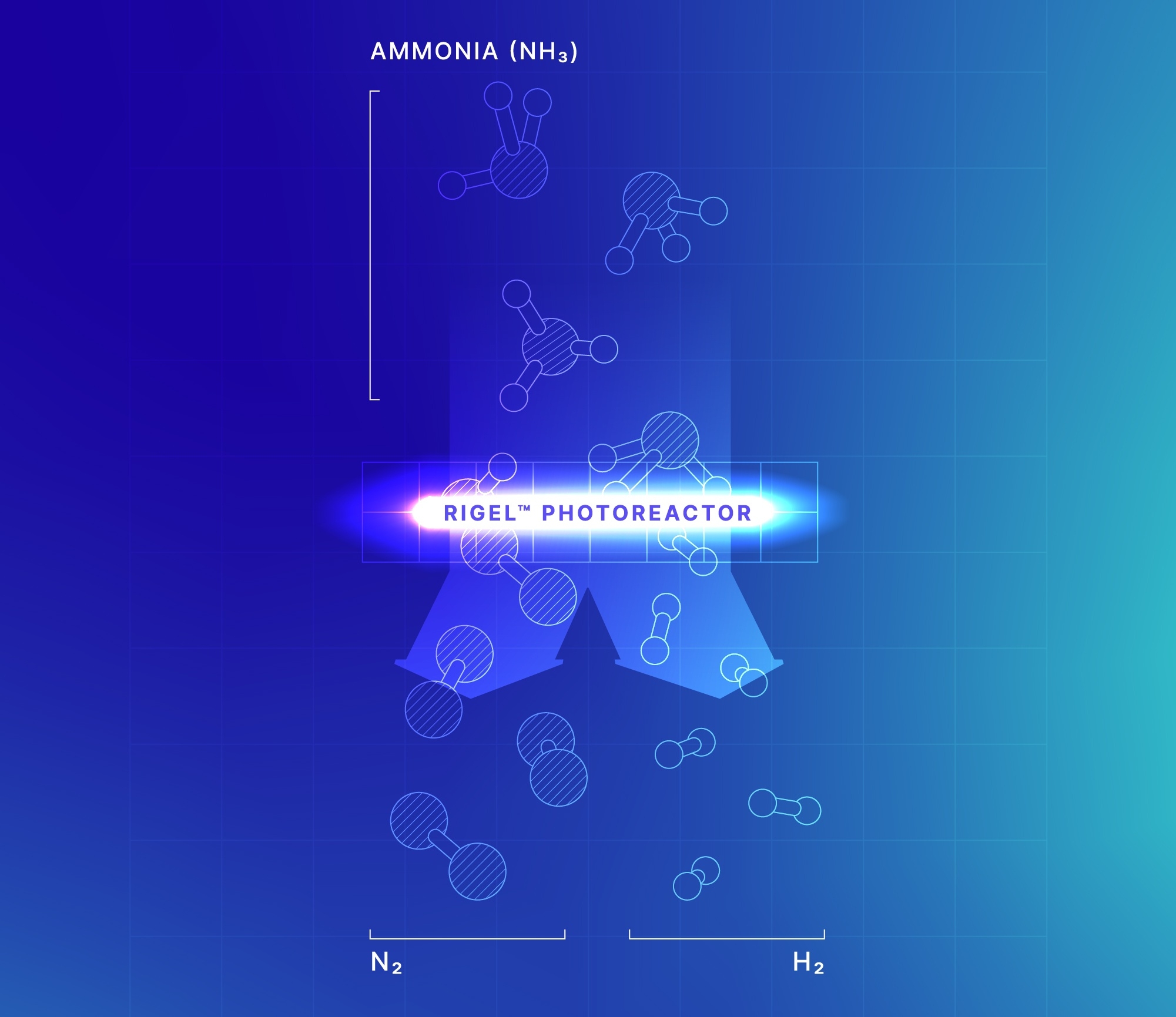
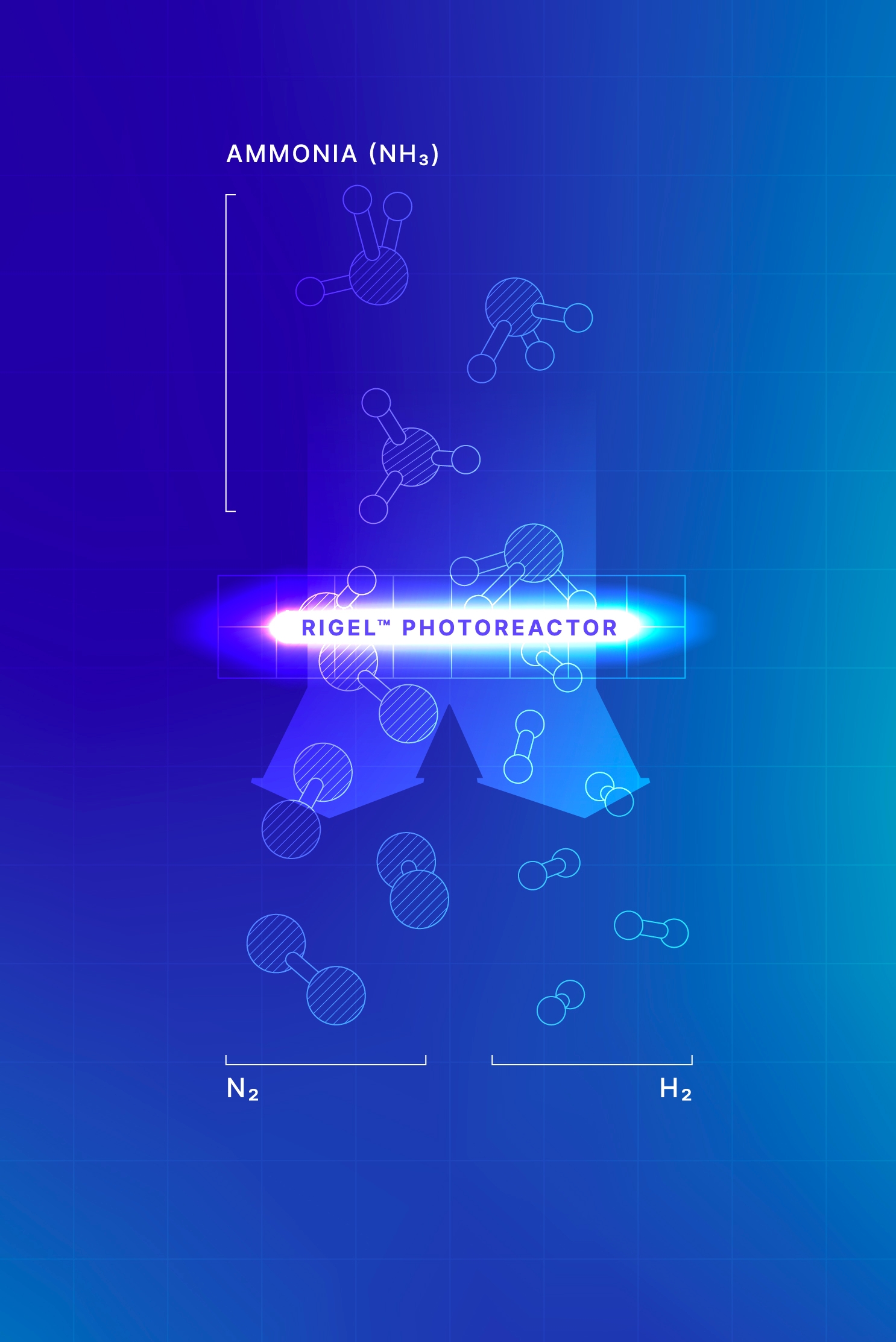
Our Ammonia e-Cracking™ for Hydrogen solution — which uses light instead of combustion as an energy source — enables the efficient and sustainable transformation of low-carbon-intensity ammonia into hydrogen when powered by renewable electricity.
This low-carbon solution gives energy importers a reliable, sustainable supply of clean hydrogen and is a key element to unlocking global hydrogen trade.
What is hydrogen used for and why does it matter?
Hydrogen (H₂) is most commonly used today to produce fertilizers, refine petroleum, and produce methanol, with emerging applications in power generation, heating, and as a low-carbon fuel. It is a strong candidate as a sustainable fuel source because it can store and deliver a tremendous amount of energy with very low carbon intensity and no emissions.
When burned, hydrogen produces only heat and water, eliminating the harmful GHG emissions produced by burning fossil fuels.
Why does low-carbon hydrogen need to be manufactured?
Very little hydrogen naturally exists on earth, so hydrogen needs to be manufactured to create compounds such as methanol.
When produced with traditional steam methane reforming, more than 10 tons of carbon dioxide are emitted for every ton of hydrogen. Using coal to manufacture hydrogen exponentially increases emissions.
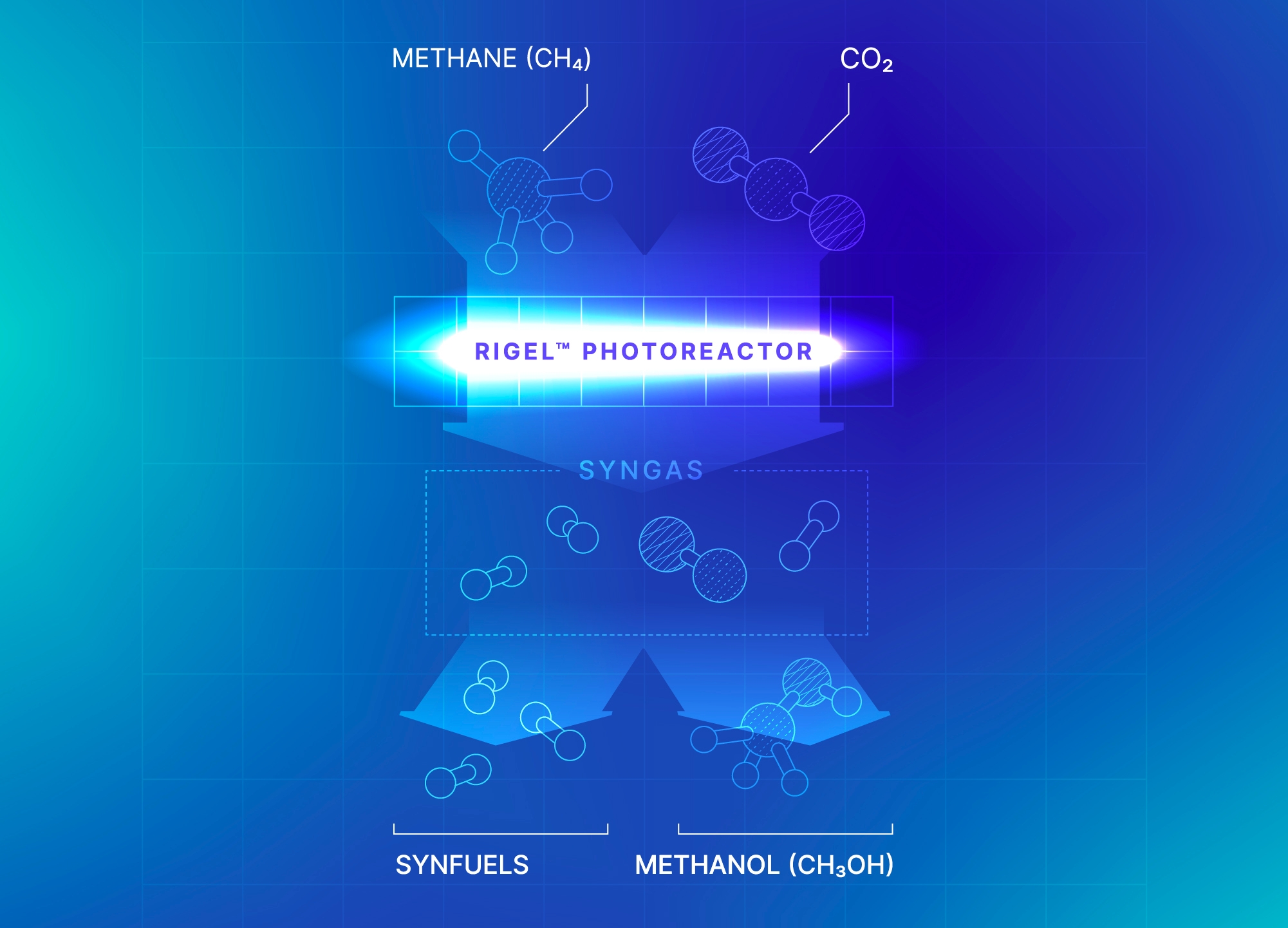
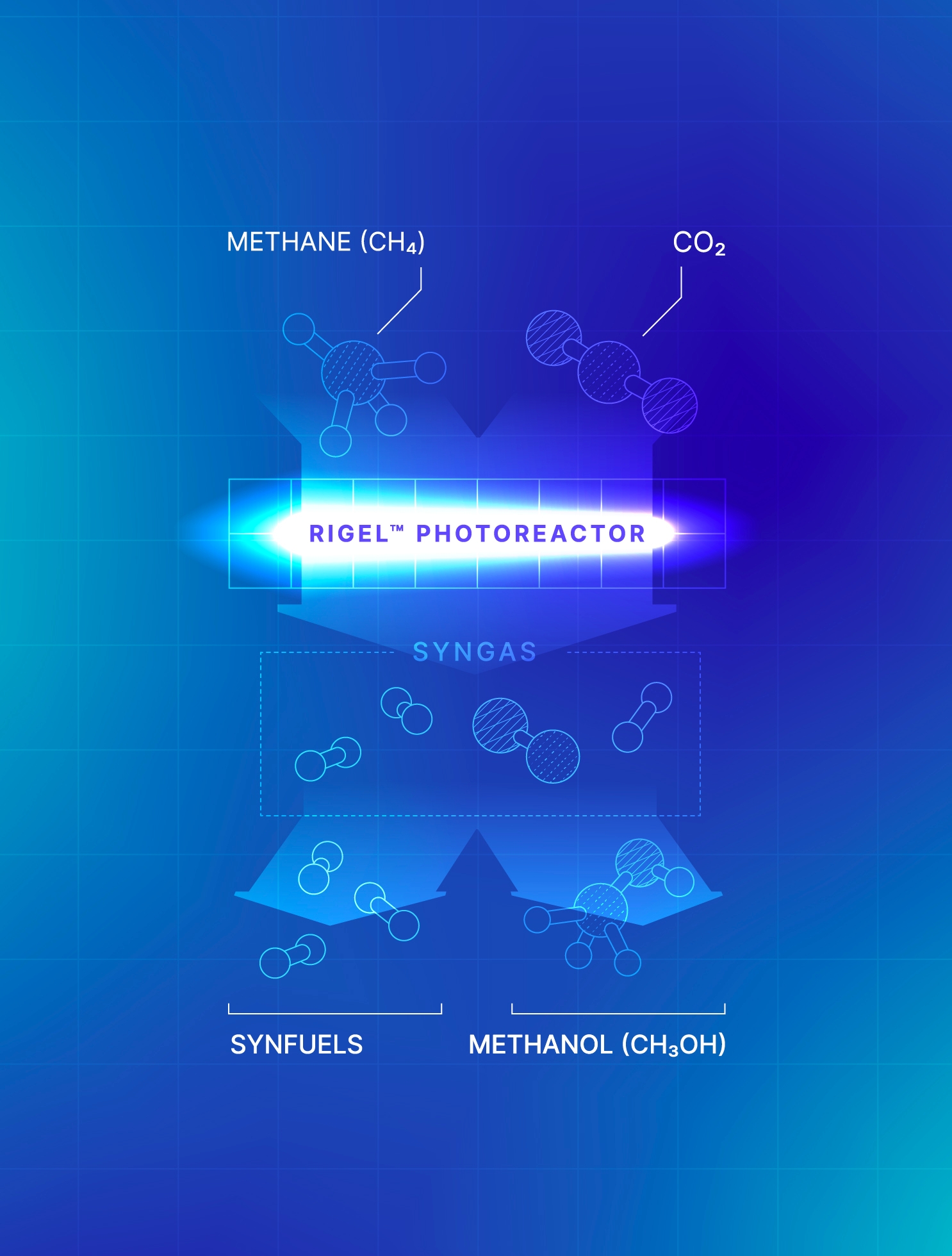
Our GHG e-Reforming™ for Syngas and Fuels solution transforms methane and CO₂ into syngas that can then be further processed into low-carbon fuels and methanol.
Our process not only replaces the cost of storing CO₂ with a valuable output, but also opens the possibility for carbon-negative fuel and methanol production when utilizing renewable biogas as a feedstock.
What is methanol and why does it matter?
While methanol is a clear liquid used in common products like plastic, paint, and cosmetics, it is also used as a relatively clean-burning and biodegradable fuel in the marine, automotive, and electricity sectors. Many see it as a strong candidate for reducing carbon intensity in heavy transport and maritime applications.
What is renewable methanol?
Syzygy can produce low-carbon-intensity methanol (renewable methanol) by combining captured carbon dioxide and methane captured from biological sources like dairies and landfills. Compared to standard fuels, renewable methanol can significantly lower carbon-dioxide and nitrogen-oxide emissions.
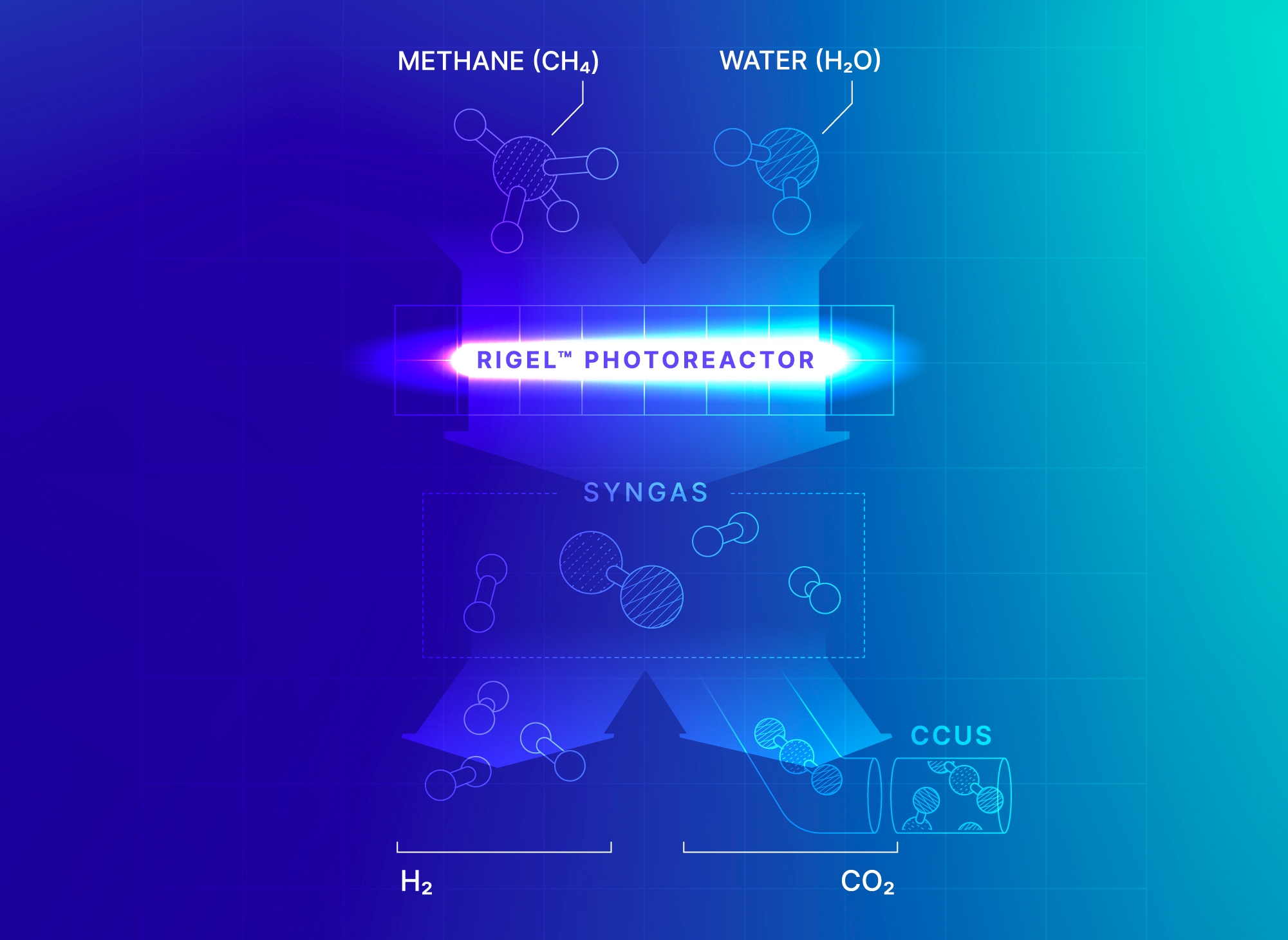
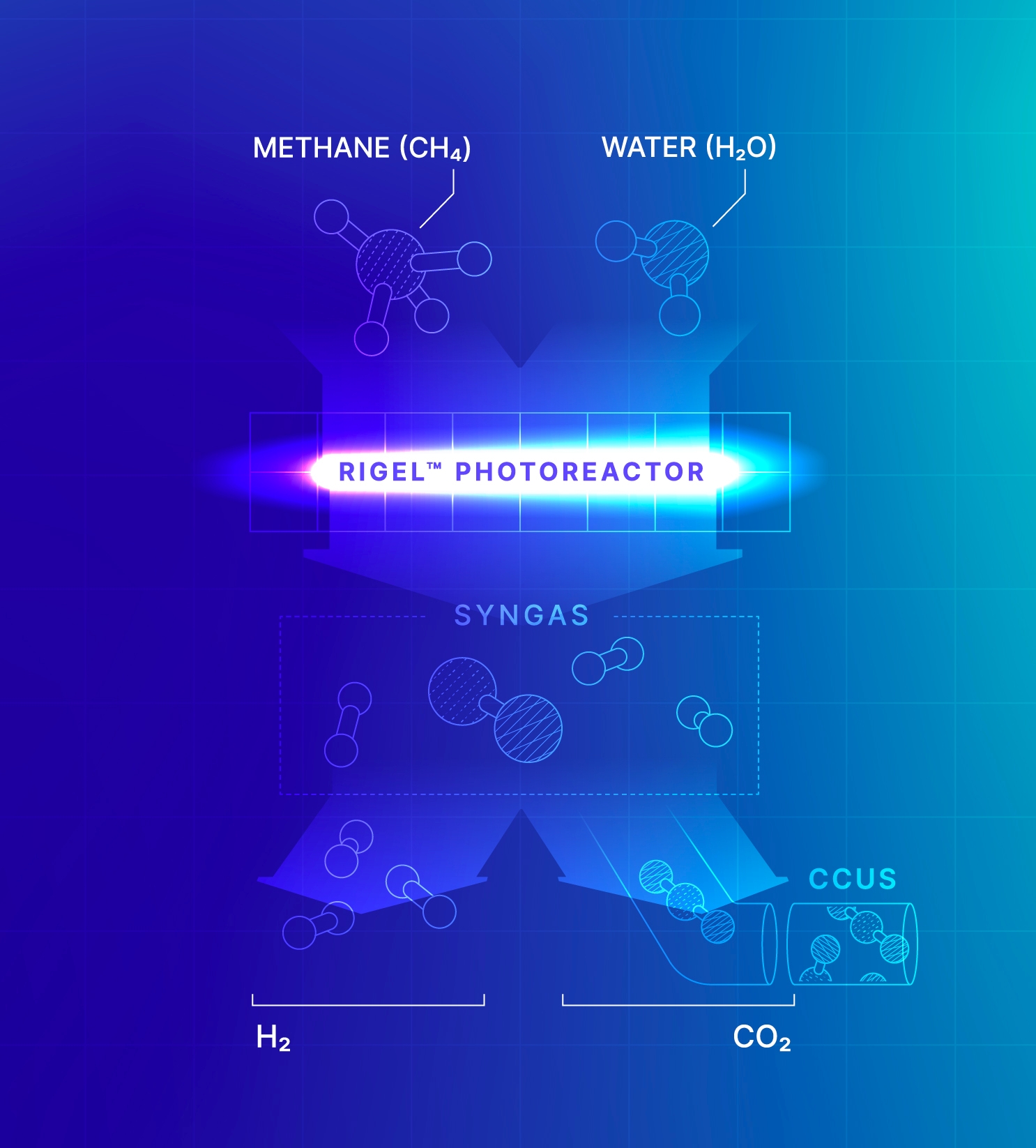
Our Steam Methane e-Reforming for Hydrogen solution provides a lower-emissions alternative to traditional greenhouse-gas-heavy steam methane reforming. Our combustion-free, all-electric Rigel™ reactor cells can be easily integrated into existing hydrogen plants to produce extra-blue
hydrogen.
Eliminating combustion from the process reduces CO₂ emissions by 40%. This solution reduces the carbon intensity of hydrogen and helps manufacturers lower operational costs. Moreover, the CO₂ produced as a byproduct of the process can be easily captured and sequestered or processed into low-carbon fuel or methanol.